Focusing on the treatment of cancers and autoimmune disorders, Usynova takes clinical needs and market demands as its orientations, and adopts key targets and their associated biological pathways as the core, trying to develop innovative and differentiated micromolecular or biomacromolecular products with global benefits. At present, the biological directions with which the company is concerned include key oncogenes, tumor immunity & microenvironment, new targets of autoimmune disorders, and refractory targets.
-Featured micromolecular pharmacochemistry platform: built an experienced internal micromolecular pharmacochemistry team and R&D platform. Possessed key R&D capabilities and techniques, from topic selection and project approval, through micromolecular drug design, chemical synthesis, structure-activity relationship (SAR) analysis, and synthesis process optimization, to biological assay and compound determination. Emphasized the tackling of important and refractory targets using special chemical and biological methods such as allosteric inhibitors, PPIs, and covalent mechanisms.
-Antibody drug R&D platform Built and perfecting monoclonal antibody and double antibody drug R&D platform. Developed key techniques and technology platforms, including monoclonal antibody development and preparation through a skillful use of mouse Hybridoma technology platform, antibody optimization and humanization, cell fermentation, and protein purification.
-Recombinant protein R&D platform: developed a recombinant protein expression and fermentation process using Escherichia coli and Pichia pastoris as carriers; completed process design from laboratory-scale to 2,000 L scale-up production; and realized the complete process route of fermentation-purification-preparation production.

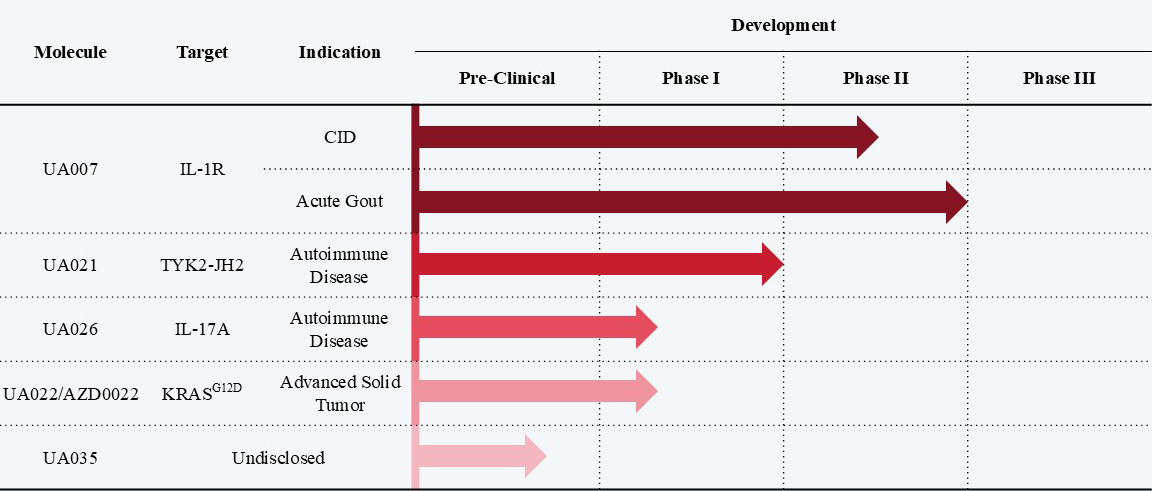
UA007 is an interleukin-1 receptor antagonist that is currently under research in two phase-II clinical studies in China. The Phase I and Phase IIa clinical studies of UA007 for the indication of CID in the treatment of colorectal cancer have been completed, and achieved POC results. The Phase I and Phase II for the indication of acute gout has been completed and achieved expected results.
UA021 is a novel, oral, selective tyrosine kinase 2 (TYK2) inhibitor, intended for the treatment of psoriasis. The Phase I studies in Australia and China have been completed and in now undergoing the Phase II study in China.
UA026 is an interleukin-17A (IL-17A) inhibitor developed for the treatment of psoriasis, and currently undergoing the Phase I study. Currently, no oral formulation targeting IL-17A has been approved globally. UA026 represents China's first oral small-molecule IL-17A inhibitor to enter clinical development, demonstrating significant potential to address an unmet medical need in this therapeutic area.
UA022, a small-molecule drug targeting KRASG12D mutations independently developed by Usynova, entered into an exclusive global licensing agreement with AstraZeneca (LSE/STO/Nasdaq: AZN) in 2023. Under the terms of the agreement, AstraZeneca has secured exclusive worldwide rights for the research, development, and commercialization of UA022.

 021-53308801
021-53308801 hr@usynova.com
hr@usynova.com Building 8, No. 88 Darwin Road, China (Shanghai) Pilot Free Trade Zone, Shanghai , China Pilot Free Trade Zone.
Building 8, No. 88 Darwin Road, China (Shanghai) Pilot Free Trade Zone, Shanghai , China Pilot Free Trade Zone.